Half-way through Digestive Disease Week 2015, I have decided to discontinue my little experiment that consisted of shortening my blogs here to a seven-sentence limit to make live-blogging feasible from the conference (see my earlier blog entry about that). In the first couple of days at DDW 2015 I was only able to squeeze in time for 2 blog entries (see below), even in the shorter format. I have concluded that things are just too busy at these conferences for me to blog meaningfully, and there is nothing I can do to change that if I want the meetings to be as incredibly productive for me as they have been. But that is why we do experiments – to learn something new. And I have learned that sharing information from DDW is only feasible for me in shorter tweet format. So I am now concentrating on highlighting key new and interesting information on FGIDs more actively on Twitter. instead Follow my stream there at @DrPalssonUNC to keep up with that. Twitter seems to be by far the most effective way for information to flow from the conference in real-time, and I will focus on participating in that information outpouring to give FGID info from the more emphasis.
DDW 2015: Quantifying the Substantial Emotional Burden of Fecal Incontinence
It is widely recognized that fecal incontinence is a distressing health problem, but there is very little information in the literature on the psychological impact of this problem in the general community.
Today, I am presenting a Poster of Distinction here at Digestive Disease Week, with the findings of a community survey of a broad sample of U.S. adults that our team conducted to assess the psychological effects of having FI.
We compared the responses of 234 adults with FI (defined as accidental loss of liquid or solid stool at least once a month in the past 6 months) to those of a control sample of 328 subjects without FI, using a set of questionnaires that included multiple measures of Fecal Incontinence, a Quality of life measure (the FI-QOL Scale) and a psychological distress questionnaire(Brief Symptom Inventory – 18; or BSI-18).
We found that people with FI had significantly higher average scores (see Figure 1 below) on all dimensions of psychological distress (anxiety, depression, somatization and overall distress) on the BSI-18 questionnaire compared to those without FI symptoms), and that substantially higher proportion of FI sufferers scored in the clinically significantly distressed range on this questionnaire (BSI-GSI scores > 63) according to guidelines for BSI-18 interpretation (see Figure 2).
High psychological distress was a significant independent predictor, along with greater severity of fecal incontinence and younger age, in predicting the amount of quality-of-life impairment among the individuals with FI.
One must always be careful in assuming causality when interpreting the findings of a single-time assessment like we used here. However, these results strongly suggest that the burden of FI symptoms results in clinically significant levels of psychological distress for a substantial proportion of FI sufferers, with the majority of individuals with FI suffering from clinical levels of psychological distress, and it further seems that having FI at a relatively younger age adds to the burden of this health problem.
Figure 1.
Figure 2.
Presentation:
Olafur S. Palsson , Steve Heymen , William E. Whitehead . Fecal Incontinence is Associated with Clinically Significant Psychological Distress. DDW 2015 Sa1366
DDW 2015: Psychological symptoms are correlated with bowel symptom severity both in IBS and IBD
A large proportion of individuals with functional GI disorders such as IBS report significant levels of psychological distress (50-90% of people with IBS in clinical samples in fact have diagnosable affective disorder – less in community samples), but it generally been unclear whether this elevation in psychological symptoms is a cause or amplifier of GI symptoms or the result of the bowel symptoms.
In contrast, individuals with bowel symptoms of more organic nature, like inflammatory bowel disease (IBD), tend to have lower levels of psychological distress than functional GI patients.
In a study presented as a poster today, Saturday May 16, Piacentino and colleagues in Rome, Italy, examined the relationship of psychological symptoms on the SCL-90_R with bowel symptom severity in 75 IBS patients and 69 patients with inflammatory bowel disease (35 of those had ulcerative colitis and 34 had Crohn’s Disease).
In line with prior studies, they found that on average the IBS patients had significantly higher scores on psychological distress than those with IBD.
However, both group scored significantly higher than population norms for psychological symptoms, and for both groups, and there was a clear increase in psychological symptom level with increasing severity of bowel symptoms.
This pattern of findings suggest that the elevated psychological distress of many GI patients is driven to substantial degree by the the gastrointestinal symptoms, regardless of whether the symptoms are organic or functional in nature – probably the result from the added stress and life interference that those gut troubles produce – although a bi-directional relationship between symptoms and psychological symptoms certainly is likely as well.
Presentation:
Daria Piacentino , Monica Cesarini , Enrico Corazziari. Gastrointestinal patients’ psychopathological level is associated with symptom severity irrespectively of inflammatory bowel disease or irritable bowel syndrome diagnosis. DDW 2015 Sa2009.
DDW 2015: Introducing My Seven-Sentence Mini-Blog Format
The last three years when I have attended the annual Digestive Disease Week, I have endeavored to share on this blog some of the many new and interesting research findings on functional GI disorders presented at that meeting – information that I think may be of interest to patients with these disorders and the general public. You can see the results of this effort in the archive here below.
Although I have been happy enough with what I have been able to write, it has troubled me each year that I have only been able to write a few posts from the meeting – far less than I would like to share with my audience. I will readily admit that this is in part because I have been somewhat verbose in my blogs. The scientist in me tends to want to explain everything in detail with facts, figures and context. That kind of writing takes a lot of time, and it simply does not lend itself very well to live-blogging from a conference. The fact of the matter is that I never have nearly enough free hours at DDW to accomplish as much as I would like with such thorough blogging. The conference days are always an exciting and extremely busy time for me, filled with numerous meetings with research colleagues, presentations and poster sessions, receptions and dinners, leaving little time for writing.
In pondering this dilemma the other day, it struck me that it might be helpful if I limited myself strictly to a self-imposed shorter blog format – turned my blogging into mini-blogging – so that I could cover more topics more quickly in my live-blogging from these meetings.
The more I thought about it, the more I liked the idea of adopting short-format blogging, as a fun writing challenge for myself for DDW 2015. After a lot of consideration about how much space I would absolutely minimally need to cover the typical topics I want to share from DDW in a reasonable but brief way, I settled on a seven-sentence limit for my new experimental format. And to make it a bit more fun as a challenge for myself, I decided to set myself the absolute rule of writing each blog topic from this year’s DDW in exactly seven sentences: No more, and no less.
So that is what I will do, and I invite you to watch this blog during DDW 2015 (May 16-19) to see how I do with my personal writing experiment. I hope that my adoption of this self-imposed mini-blog format will enable me to share more of the wealth of interesting new FGID content that I learn about at the meeting in a readable and understandable manner. I will certainly welcome any feedback on this approach. And who knows? If it works well, it might even become my standard way of writing this blog from now on.
DDW 2014 Note: Caraway Oil Poultices as IBS Treatment
I always find it intriguing and encouraging when herbal and alternative medicine treatments that I have never known to be used for IBS are tested and show good promise in research studies. Yesterday, May 3 2014, a team of researchers from Essen, Germany presented the results of one such study at Digestive Disease Week 2014; a randomized controlled trial that indicates that poultices with caraway oil might be beneficial for reducing IBS symptoms and improving the quality of life of IBS sufferers. A poultice an old-fashioned time-honored form of treatment — a soft moist mass containing therapeutic herbal or chemical ingredients that is applied to the surface of the body. Poultices are for example often used to relieve pain, inflammation or swelling. I knew that caraway is an old and very useful medicinal plant with digestive benefits, from growing up in Iceland where it is cultivated and used (among other things to flavor the national strong Vodka-like spirit informally called Black Death). I had never known caraway oil to be used in poultices, however, or for IBS treatment.
The German research team conducted a well-designed trial. They treated 48 IBS patients with diarrhea-predominant and alternating bowel symptoms, and compared daily use of hot poultice of caraway oil for three weeks to daily use of hot or cold poultices with olive oil instead, each also for three weeks. Every patient used both the caraway treatment and the comparison interventions, in a random order, with a two-week recovery (wash-out) period between different treatment conditions.
The results clearly favored the hot caraway oil over the comparison interventions. Symptom severity was reduced much more on average from that treatment than the others, and a higher proportion of patients was deemed treatment responder with the caraway oil (43.9%) compared to hot (20.0%) or cold (18.9%) olive oil. Additionally, health-related quality of life, as measured by the IBS-QOL scale, improved significantly more with the caraway oil than the olive oil poultices.
In short, the results of this study seem to me to show tantalizing promise of a potentially valuable therapy for IBS patients. The main concern I have about this study is the possibility that caraway oil, which I believe has a sharp spice scent, may have seemed more “medicinal” to the patients than the olive oil interventions and therefore may have raised expectations of benefit and thereby caused a stronger placebo response than olive oil. However, the researchers mentioned in their abstract that they made adjustments for expectancy in their statistical analyses, so perhaps this was taken into account. I think it would be great to see further good-quality research done on these hot caraway oil poultices as possible useful therapy for IBS.
Presentation:
Sa1071 Jost Langhorst,Romy Lauche ,Anke Janzen, Rainer Lüdtke, Holger Cramer, Gustav J. Dobos. Efficacy of Caraway Oil Poultices in the Treatment of Irritable Bowel Syndrome – a Randomized Controlled Cross-Over Trial. Departments of Integrative Gastroenterology and Internal and Integrative Medicine, University Duisburg-Essen, Essen, Germany; Karl and Veronica Carstens-Foundation, Essen, Germany
DDW 2014 Note: Altered Balance of Bacteria in the Mouths of People With IBS.
Advances in technology and ever-decreasing cost of large-scale DNA analysis have made it possible in the past few years to conduct a census of the bacterial populations that live in different places inside individual human beings. A number of such studies have been done on IBS patients, and they are revealing that IBS patients may have an abnormal balance in their bacterial populations (more of certain bacteria types and less of others) compared to other people. This raises the possibility that such bacterial imbalance may be contributing to symptoms and, by extension, that these bacterial differences could be corrected to help improve IBS. And in fact, both treatment with probiotics (beneficial bacteria that are taken in capsules and are thought to restore balance in the bacterial population in the intestines) and treatment with antibiotics that mostly work within the bowel (neomycin and rifaximin) have shown clear positive effects on the symptoms of some IBS patients in multiple studies1,2. This provides indirect but persuasive evidence that bacteria play a definite role in IBS symptoms. However, much is still poorly understood about this phenomenon of bacterial abnormality in IBS. It is unclear, for example, which bacteria exactly are trouble-makers (apart from a couple of notorious offenders like Clostridium Difficile) or how they cause trouble.
The balance between different types of bacteria in IBS has typically been studied in stool samples, and that makes good sense since IBS is a bowel problem. But could bacterial imbalances also be present in other parts of the body in people with IBS? A study presented yesterday (Saturday May 3) at Digestive Disease Week 2014 in Chicago indicates that bacterial abnormalities are in fact present in the other end of the GI tract as well – in the mouths of IBS patients.
Fourie and colleagues at the National Institutes of Health in Bethesda, Maryland, collected samples of bacteria from the lining of the mouth cavity of 19 IBS patients and also from the same number of healthy controls who were matched to the IBS patients in height, weight, race and sex. They then ran whole microbiome DNA analysis on the samples to identify the types of bacteria present based on their genes, and then calculated how abundant different types of bacteria were in the mouths of IBS sufferers versus the healthy comparison subjects.
The team found that the people with IBS had significantly more of three bacteria types – Prevotellacea, Lachnospiraceae, and Rikenellaceae – compared to the healthy individuals. The latter two kinds of bacteria have already previously been found to be incresed in IBS according to the authors. The researchers further did some bowel stress test that involved swallowing a mixture of 4 types of sugars, and measured the stress hormone cortisol in the participants’ urine to evaluate the amount of stress response to this stimulation, and they discovered that the amount of Lachnospiraceae bacteria was related to the intensity of the body’s stress response to the sugar stress test. However, IBS patients (who had a higher concentration of that type of bacteria) had less stress response to the sugar stressor, so the meaning of that bacterial association is a bit ambiguous.
The significance of this study, even though it is small and the findings need to be replicated, is that it indicates that abnormal bacterial balance is probably not limited to the lower part of the GI tract in IBS, but is likely to be found all over in the GI tract – even in the parts furthest away from the small or large bowels where most biological and physiological investigations in IBS have been concentrated. As the authors suggest, sampling bacteria in the mouth could be a convenient way (compared to stool samples) to measure the microbe inbalances in IBS patients that could potentially be treated to improve their IBS symptoms.
Presentation:
Sa1185. Nicolaas H. Fourie, Dan Wang, Paul A. Smyser, Sarah Abey1, LeeAnne Sherwin, Bridgett Rahim-Williams, Wendy A. Henderson. Dysbiosis of the Mucosa-Adherent Microbiome in Patients With Irritable Bowel Syndrome. NINR & NIMHD, NIH DHHS, Bethesda, MD.
References:
1. Ohman L, Simrén M. Intestinal microbiota and its role in irritable bowel syndrome (IBS). Curr Gastroenterol Rep. 2013 May;15(5):323. Review. PubMed PMID: 23580243
2. Cash BD. Emerging role of probiotics and antimicrobials in the management of irritable bowel syndrome. Curr Med Res Opin. 2014 Apr 14. [Epub ahead of print] PubMed PMID: 24666019
DDW 2014 Note: How Common is Functional Dyspepsia in U.S. Adults? Findings From Our National Survey.
Functional dyspepsia (FD) is a common disorder characterized by symptoms that are often described as indigestion; unpleasant sensations in the middle of the abdomen, uncomfortable fullness after eating, and sometimes inability to eat normal-size meals. FD is a much-researched disorder, but surprisingly, no nationwide studies have been done in the U.S. to evaluate what proportion of adults actually meet formal criteria for FD as defined by the current Rome III diagnostic criteria. Moreover, little is known about how rates of dyspepsia differ among race or ethnic groups or between different age group in the American population.
Today (May 3, 2014) I presented in poster format at Digestive Disease Week 2014 in Chicago the results of statistical analyses that our research team carried out to estimate how common FD is in American adults. We used for this purpose data from the Rome Normative Gastrointestinal Symptom Survey that we conducted last year, and which was sponsored by the Rome Foundation. In this secure online survey, we obtained responses from a large group of adults across every state in the U.S. The survey included questions about the responders’ demographics, health history, and the whole Rome III Diagnostic Questionnaire that diagnoses all functional gastrointestinal disorders. To minimize bias due to the possibility that people with GI symptoms might be more interested than others in participating and therefore be over-represented in our sample, invitations to participate described the study as a physical symptoms survey but did not mention gastrointestinal symptoms. We also controlled how many participants in different demographic categories could participate, in order to ensure equal gender proportions and get enough minority participation for our sample to be similar to the U.S. population.
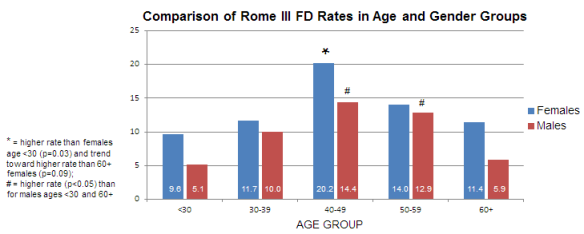
A total of 1,665 individuals ranging in age from 18-94 years completed our survey, but we then eliminated from our analysis dataset people who showed evidence of inconsistent survey answers (we included three repeat questions in the survey for quality-check purposes), leaving us with responses from 1,277 people suitable for analysis. We have used that sample to estimate how common different functional gastrointestinal disorders are in the U.S. population and whether they have different frequency in various subgroups of the population (like males vs. females or older individuals versus young). In today’s presentation we only reported such findings for FD, but we will also be presenting our results for IBS and fecal incontinence separately here at DDW on Tuesday. We found that 11.4% of the people who completed our survey met Rome III criteria for FD. However, to get a more accurate estimate of the national dyspepsia prevalence, we statistically adjusted (weighted) our calculations to make the sample match the national adult age, gender and race/ethnicity distribution in the 2010 U.S. Census. Doing this only altered our numbers slightly, resulting in an estimated overall national U.S. FD rate of 11.9%.
We also found that functional dyspepsia tended to be more common in women than men (13.0% versus 9.9%) but this actually did not quite reach statistical significance, so we cannot be confident about the sexes really being different in this regard. In both sexes, FD rates were lowest in the youngest and oldest age groups, and significantly higher at mid-life; see the Figure below. Dyspepsia prevalence was not significantly different in white (12.3%) hispanic (12.9%) and black (9.6%) respondents in our survey.
There are presently two recognized subtypes of FD, separated by difference in the symptom pattern. A surprising finding in our survey was that only 3 individuals in our whole sample (a mere 0.2%) met criteria for one of those sub-types, which is called Epigastric Pain Syndrome (defined primarily by pain in the upper gut, above the stomach). In contrast, most of the people (87%) in our survey who qualified for FD diagnosis based on their responses fit into the other subgroup, called Postprandial Distress Syndrome (meaning that they mostly have uncomfortable fullness after eating).
In summary, our analyses of responses to the FD diagnostic questions in this large nationwide survey give a clear picture for the first time of the prevalence of this disorder overall in the U.S. and in population subgroups, as it is defined by current Rome symptom criteria. Our results indicate that about 12% of American adults have this disorder, which makes it one of the most common of all gastrointestinal problems in the U.S.
Presentation:
Sa1335. Olafur S. Palsson, Miranda A. Van Tilburg, Brennan M. Spiegel, Jan F. Tack, Robin C. Spiller, Lynn S. Walker, Yunsheng Yang, William E. Whitehead. Uninvestigated Dyspepsia in the U.S. General Population: Results From the ROME Normative Gastrointestinal Symptoms Survey (RNGSS). Center for Functional GI and Motility Disorders, University of North Carolina, Chapel Hill, NC; Digestive Diseases, VA/UCLA, Los Angeles, CA; TARGID, University Hospitals of Gasthuisberg, Leuven, Belgium; Nottingham Digestive Diseases Centre Biomedical Research Unit, University of Nottingham, Nottingham, United Kingdom; Adolescent Medicine, Vanderbilt Children’s Hospital, Nashville, TN; Department of Gastroenterology and Hepatology, Chinese PLA General Hospital, Beijing, China.
DDW 2013 Note: Getting a Clear Picture of the Average Symptom Pattern in IBS
Irritable bowel syndrome (IBS) is a disorder that is defined entirely in the formal diagnostic criteria (currently the Rome III criteria) by the symptoms of abdominal pain, diarrhea and/or constipation. All of these three symptoms are known to be intermittent in the disorder. However, because few studies have tracked the bowel symptoms of large groups of IBS sufferers carefully on a daily basis over extended periods of time, our knowledge about the typical episodic behavior of these symptoms has been poor, and surprisingly little has been known about to what degree the different bowel symptoms occur together or separately. How often do patients for example have constipation on days when they also have abdominal pain? And what proportion of their total days do they have bowel symptom episodes of any type? This kind of information has not been available in IBS.
Today I presented the results of a study by our UNC-Chapel Hill research team at Digestive Disease Week 2013 in Orlando which aims to give a better picture of the typical symptom episode pattern in IBS overall. We analyzed diary data from 124 adult IBS patients who met the Rome III diagnostic criteria for the disorder and had also been diagnosed by a doctor as having the disorder. These study participants tracked their every bowel movement (BM) for 90 days in a pocket-sized diary. They rated the consistency of each bowel movement on the Bristol Stool Scale (1-7 ratings with verbal and picture descriptors where 1-2 is considered constipation and 6-7 diarrhea: See more about the scale here) and transferred these ratings to a secure website each night, where they also rated global 24-hour abdominal pain (0-10 scale).
Since we wanted to study typical symptom activity in IBS, we did several things to try to ensure that the diary data in our study reflected as natural and typical IBS symptoms as possible:
- We used a long diary recording period. IBS is a fluctuating disorder, so to capture a characteristic pattern of symptoms for each individual, we decided we would have to track the symptoms for months. We therefore decided on a three-month (90-day) diary recording.
- We did not study patients who were coming to clinics or doctors for help, because those would be likely to have more active symptom episodes that were bringing them in at the present time. Instead, we advertized for patients who had been diagnosed with IBS through our University mass e-mail system, on various websites and also recruited participants who had signed up in a registry to participate in research studies for our Center. We then verified that they met Rome III criteria for IBS.
- We only included in our analysis diary information from patients who reported no laxative or anti-diarrheal medication use in their diaries during their whole diary recording period, and who were on none of the IBS-specific medications, so we could be sure that we were seeing “natural” symptom patterns unaffected by bowel medications.
The patients who ended up included in our analysis dataset were predominantly female (89%), of an average age of 36.7 years, and were of all subtypes of IBS (38 IBS-D, 69 IBS-M, 16 IBS-C, and 1 IBS-U).
The first thing we had to do in order to be able to systematically analyze symptom episode patterns in the diaries was to define what constituted an episode for each kind of symptom. For pain episodes, this was easy – we used definitions that had already been established 1 by our colleagues in a different study, and which we found reasonable based on our data. For diarrhea and constipation episodes, however, no such episode definitions existed so we conducted systematic pattern analysis of bowel movements in all the diary datasets to come up with effective episode definitions. I will not describe those analyses here, for they are rather complicated and will be described in our forthcoming paper. But the episode definitions we ended up using for the three type of central symptoms in IBS was as follows:
- A diarrhea episode is a series of at least 2 diarrhea BMs never separated by more than 1 non-diarrhea BM nor by a day without a BM.
- A constipation episode is a series of at least 2 constipation BMs and/or one or more sequences of 3+ no-BM days, never separated by more than 1 non-constipation BM. Constipation episodes included non-BM days.
- A pain episode is a series of one or more days of daily pain severity rated higher than 3 on a 0-10 scale
Then we analyzed the diary records of the 124 IBS patients to see how long, how frequent and how overlapping or separate episodes of different symptoms were.
What we found was that on average, diarrhea episodes lasted 2.1 days, constipation episodes 4.45 days for constipation, and pain episodes 3.1 days.
Diarrhea episodes were present on 16.2% of total diary days, constipation episodes on 22.5% and pain episodes on a whopping 44.6% of days. Interestingly, pain episode days co-occurred far more frequently with diarrhea episode days than with constipation episode days (70.5% vs. 39.1% overlap).
Amazingly to me, we found that IBS symptom episodes of any kind (that is, either pain or constipation or diarrhea or a combination of more than one of these) were on average present on nearly two-thirds of all days for this sample of patients (63.8% of days to be precise). We also found (which perhaps does not surprise anybody) that the higher the percentage of their total days the patients spend in IBS symptom episodes, the poorer are their their quality of life scores.
Since this is a lot of complicated information with a whole bunch of number, a picture is worth a thousand words, as the saying goes. So here is a pie chart representing the typical symptom episode activity of the IBS patients in our study. The whole circle represents all days for the average IBS patient, and the various slices of the pie show what percentage of total days different types symptom episodes are occurring.
Figure. Average Symptom Pattern in IBS (% of total days; based on 3-month diaries of 124 patients):
We are continuing to analyze these diary data to further understand symptom patterns and will soon be describing our findings in more detail than we did here at DDW today in our forthcoming paper. Of course there is a huge amount of variability between individuals in their symptom picture, so the figure above does not apply to all IBS patients. Nonetheless, I think it is useful to depict the symptom episode pattern overall across IBS subtypes as I have done here, so that one can get a better appreciation of the general nature of IBS symptom activity. For example, I was surprised to see how much of total days in the life of IBS patients symptom episodes are present, even though I have done research and clinical work with IBS for nearly 20 years. You cannot get that type of bird’s-eye summary view of what IBS looks like except through this kind of thorough symptom-tracking in a large number of individuals across a long period of time.
In summary, what we are finding is that the three defining symptoms of IBS all occur in distinct episodes rather than being randomly spread over time. Pain episodes are the most frequent type of symptom episode, roughly happening on average on 2 out of every 5 days in the life of the typical IBS patients, and these pain episodes tend to overlap with diarrhea episodes more commonly than with constipation episodes. On average, patients experience IBS symptom episodes on nearly 2/3 of all days. More frequent IBS episode days are associated with increased quality of life impairment.
Presentation:
Tu2070. Symptom Episode Patterns in Irritable Bowel Syndrome (IBS). Olafur S. Palsson, Jeffrey S. Baggish, William E. Whitehead. Center for Functional GI and Motility Disorders, University of North Carolina, Chapel Hill, NC and CSL Behring, King of Prussia, PA
References:
1: Hellström PM, Saito YA, Bytzer P, Tack J, Mueller-Lissner S, Chang L. Characteristics of acute pain attacks in patients with irritable bowel syndrome meeting Rome III criteria. Am J Gastroenterol. 2011 Jul;106(7):1299-307. doi: 10.1038/ajg.2011.78. Epub 2011 Mar 29. PubMed PMID: 21448146.
DDW 2013 Note: Unusually High Rate of Joint Hypermobility Syndrome in Patients Diagnosed with Functional GI Disorders
Joint Hypermobility Syndrome is a connective tissue disorder characterized by overly elastic skin and “loose joints” that bend too much (joint hyperflexibility)1. It is a fairly common condition (it may be present in as many as 15% of the population) that has genetic underpinnings and runs in families, but it is generally benign and typically goes undiagnosed because in many people it does not produce any troublesome symptoms (this should not be confused with a more serious but rare condition with somewhat similar connective tissue abnormalities, called Ehlers-Danlos Syndrome). Because the joints are so loose in people with Joint Hypermobility Syndrome, those individuals are prone to joint dislocations, sprains and painful strain around their joints. When symptoms do occur, these are therefore often pain in joints – fingers, elbows, knees and fingers. In recent years, a couple of studies in the United Kingdom have indicated that gastrointestinal symptoms are associated with this syndrome. One study found that patients with this connective tissue syndrome had greater constipation and rectal evacuation problems than other people2, and another found higher than expected prevalence of this syndrome in a retrospective survey of patients with unexplained GI symptoms who had been seen in a neurogastroenterology clinic3.
Yesterday, Sunday May 19, the same team of researchers from the United Kingdom who published those previous observations presented a new well-designed study at Digestive Disease Week 2013 in Orlando that gives a clearer and more definite picture of the association of Joint Hypermobility Syndrome with gastrointestinal problems. They evaluated 694 newly referred patients in a row who were coming to gastroenterology (GI) clinics, and used the so-called Brighton Diagnostic Criteria to assess whether they had Joint Hypermobility Syndrome. They also evaluated 92 patients in other clinics coming for non-GI problems for comparison. After the GI patients had been evaluated and diagnosed by the GI doctors they were seeing, the researchers compared the prevalence of Joint Hypermobility Syndrome in patients with functional GI disorders, organic GI disorders (like inflammatory bowel disease or cancer), and in the non-GI control patients. They excluded GI patients who were diagnosed with reflux disease (GERD) from analysis for a clearer picture, as GERD can be considered a mixture of functional and organic disease.
The results were interesting and thought-provoking. The rate of Joint Hypermobility Syndrome was significantly higher in patients who GI doctors diagnosed as having functional GI disorders (40.5%) than in either patients found to have organic GI disease (26.9%) or non-GI comparison patients (25%). This strongly suggests that a subset of patients who are seen by gastroenterologists and diagnosed as having functional GI disorders like IBS or functional dyspepsia actually have a verifiable organic problem – a connective tissue abnormality – that either contributes significantly to their symptoms or might even cause them altogether, in which case they are really being mis-classified as functional GI patients. These very interesting results from a well-designed study definitely warrant future examination in research on functional GI patients in my opinion, especially since the Brighton Diagnostic Criteria can be easily incorporated in questionnaire evaluation of these patients.
So how do you know if you have Joint Hypermobility Syndrome? Some signs of it are1 (1) that you are (or ever have been able to) put your palms flat on the floor standing up with your legs straight; (2) that you can (or ever could) touch your forearm with your thumb (on the same side, of course!); (3) have been known to be “double jointed” or able to contort yourself into unusual shapes to amuse and amaze your friends, or (4) often dislocated your joints as a child. And if you are a professional contortionist, it’s probably a fairly sure bet that you have this syndrome!
Presentation:
Asma Fikree, Rubina Aktar, Lucy E. Glasgow, Katherine V. Gillespie, Adam D. Farmer, Rodney Grahame, Joan K. Morris, Charles H. Knowles, Qasim Aziz. The Association Between Functional Gastrointestinal Disorders and the Joint Hypermobility Syndrome – Connective Tissue Is the Missing Link! Wingate Institute of Neurogastroenterology; Blizard Institute of Cell and Molecular Science, Barts and the London School of Medicine and Dentistry, Queen Mary University London, London, United Kingdom; National Centre for Bowel Research and Surgical Innovation, Blizard Institute of Cell and Molecular Science, Barts and the London School of Medicine and Dentistry, Queen Mary University London, London, United Kingdom; Wolfson Institute of Preventive Medicine, Barts and the London School of Medicine and Dentistry, Queen Mary University London, London, United Kingdom; Rheumatology, University College Hospital, London, United Kingdom; Gastroenterology, Shrewsbury and Telford NHS Trust, London, United Kingdom
References:
1. Simpson MR. J Am Osteopath Assoc September 1, 2006 vol. 106 no. 9 531-536. Available in full online here: http://www.jaoa.org/content/106/9/531.full
2. Mohammed SD, Lunniss PJ, Zarate N, Farmer AD, Grahame R, Aziz Q, Scott SM.
Joint hypermobility and rectal evacuatory dysfunction: an etiological link in
abnormal connective tissue? Neurogastroenterol Motil. 2010 Oct;22(10):1085-e283.
doi: 10.1111/j.1365-2982.2010.01562.x. Epub 2010 Jul 5. PubMed PMID: 20618831.
3. Zarate N, Farmer AD, Grahame R, Mohammed SD, Knowles CH, Scott SM, Aziz Q.
Unexplained gastrointestinal symptoms and joint hypermobility: is connective
tissue the missing link? Neurogastroenterol Motil. 2010 Mar;22(3):252-e78. doi:
10.1111/j.1365-2982.2009.01421.x. Epub 2009 Oct 15. PubMed PMID: 19840271.
DDW 2013 Note: Yes, You Can Get IBS and Functional Dyspepsia from Bad Drinking Water
In December of 2010, a fire broke out in an apartment building in Belgium. As firefighters struggled to contain the blaze, they used sewer water to fight the fire. In the process, they accidentally connected that water source to the main community water supply. Sewer water, containing among other things infectious organisms like norovirus, Giardia and Campylobacter, was pumped into the drinking water pipes and spread quickly throughout the water supply of this town of about 18.000 people. Within 24 hours, a lot of people were seeking help for acute gastrointestinal illness, and it was clear that a major outbreak of GI infection had occurred.
It is now well known from many studies that a subset of people who become sick from food poisoning or contaminated water develop chronic functional gastrointestinal disorders as a result – irritable bowel syndrome (IBS) and functional dyspepsia (FD). Researchers are always looking for natural experiments or accidents like this water contamination to study how infections turn into chronic gastrointestinal disorders and who develops such problems and who does not. In this case Belgian gastroenterology researchers in Leuven, which happens to be a mere half-hour drive from where this outbreak occurred, were quick to descend upon the unfortunate town in order to get baseline data from the local residents and start following their health over time.
Today at Digestive Disease Week 2013 in Orlando, those researchers presented their findings of their study of the long-term effects of this massive but brief exposure to contaminated drinking water.
In the study, they asked all the residents of the affected town to complete questionnaires on gastrointestinal symptoms they had before, during and after outbreak, and also about their psychological symptoms and other characteristics. These questionnaires were completed 0-3 months after the outbreak. They also asked the subjects to complete such questionnaires again a year after the outbreak to evaluate the long-term effects on GI symptoms. A total of 1377 people completed the questionnaires, but data from children younger than eighteen and from all people who reported that they had GI symptoms consistent with IBS or functional dyspepsia before the outbreak were eliminated from analysis. This left 1028 questionnaire sets to be analyzed. It turned out that 30% of the subjects had become sick with infectious gastroenteritis within 2 weeks of the contamination. When those individuals were surveyed a year later, 15.6% of them were found to have IBS and 19% had functional dyspepsia, according to Rome III diagnostic criteria (remember, none of these people had these disorders before the gastroenteritis outbreak). Interestingly, a fairly significant number of individuals in the other 70% of the survey sample — the ones who were exposed to the contaminated water as well but did not have immediate GI problems — were also found to have IBS (6.5%) and FD (12%) after a year (much higher rates of new incidence of these disorders than one would typically expect in the general population). The investigators found that of all the individual characteristics that they assessed in the study, being of younger age and having higher somatization score at the time of the outbreak (that is, already having a lot of non-gastrointestinal body symptoms) were factors that predicted greater likelihood of developing long-term IBS or functional dyspepsia symptoms after exposure to the contamination. Having diarrhea symptoms for several days after exposure was also a risk factor for meeting diagnostic criteria for IBS a year later.
This study is a very nice addition to the research knowledge base on post-infectious IBS and post-infectious functional dyspepsia. I particularly liked that the researchers studied functional dyspepsia and IBS in parallel, for this allows direct comparison of the relative risk of developing each of those disorders long-term from infection. Another valuable aspect of the study is that the contamination here was a broad cocktail of common infectious organisms that can cause GI problems. This makes the findings more generalizable, for it shows us the substantial long-term health risk associated with drinking unclean water for even a relatively short period of time. The risk factors identified as causing people to be more likely to develop long-term post-infectious gastrointestinal diseases in this research are the same as have been found in multiple previous studies1 so this work confirms once again that younger adults are more vulnerable, and that not recovering quickly from the initial gastroenteritis bowel distress and being prone to having a lot of body symptoms are all factors make people more likely to develop chronic functonal GI disorders from infection.
I found two additional aspects of the findings from this study particularly interesting: The first was to see that people can develop long-term functional GI disorders as a result of drinking contaminated water without having any acute GI symptoms soon after the exposure to clue you in that they have even been infected at all. And the second noteworthy observation was that exposure to water contamination mostly resulted in either IBS or functional dyspepsia a year later, but few people developed both disorders: There was only 17% overlap according to my conversation with the principal author, Sander van Wanrooij, at DDW today.
Presentation:
Su2031. Postinfectious Irritable Bowel Syndrome and Functional Dyspepsia Following an Outbreak of Tap Water Contamination. Sander van Wanrooij, Mira M. Wouters, Stephanie Mondelaers, Laura van Gerven, Annick de Vries, Pedro J. Gomez-Pinilla, Guy E. Boeckxstaen. University Hospital and University of Leuven, Belgium
References:
1. Ghoshal UC, Ranjan P. Post-infectious irritable bowel syndrome: the past, the
present and the future. J Gastroenterol Hepatol. 2011 Apr;26 Suppl 3:94-101. doi:
10.1111/j.1440-1746.2011.06643.x. Review. PubMed PMID: 21443719.